Home > Sections > Elements & the Periodic Table > Atoms
Last Updated: 14th June 2023
ARCHIVED ITEM: this page is no longer updated.
Atoms
Keywords
Atoms, elements, mixtures, compounds, periodic table, smallest component, matter, nuclear fission, positive charge, negative charge.
Introduction
If you've looked at my biology pages on the site, you'll know that the smallest item in our body is a single cell. Some organisms are even single-celled. But is there anything smaller? To put simply, yes. The atom.
What is an Atom?
An atom is the smallest unit an element can be made from. Everything is made from atoms as well. That's right, you're not just a eukaryote, you're also an atom!
The technical terminology states that an atom is the smallest unit where matter can be divided. If it is split any further, and thus smaller, we get what is known as nuclear fission, which is a massive release of energy.
In chemical terms, it is the basic building block of everything. Most of the atom itself is made up of empty space. But it has a simple layout otherwise, having a nucleus, and electrically charges ions called protons, neutrons and electrons.
Here is a YouTube video from Cognito to explain more about atoms:
History
While the atom has been studied for decades, no real picture has been able to have been taken, so physicists have had to "best-guess" what they look like, and so complimentary images of the make-up of an atom are made.
Structure
Despite different atoms having different amounts of electrons and protons, they are all still the same size. To put an atom's size into perspective, if you lined 50 million atoms in a row, they would only equate to 1cm (0.4 inch) in length.
Interesting fact: everything in the universe is made up of atoms. Literally. An atom is the smallest unit of matter.
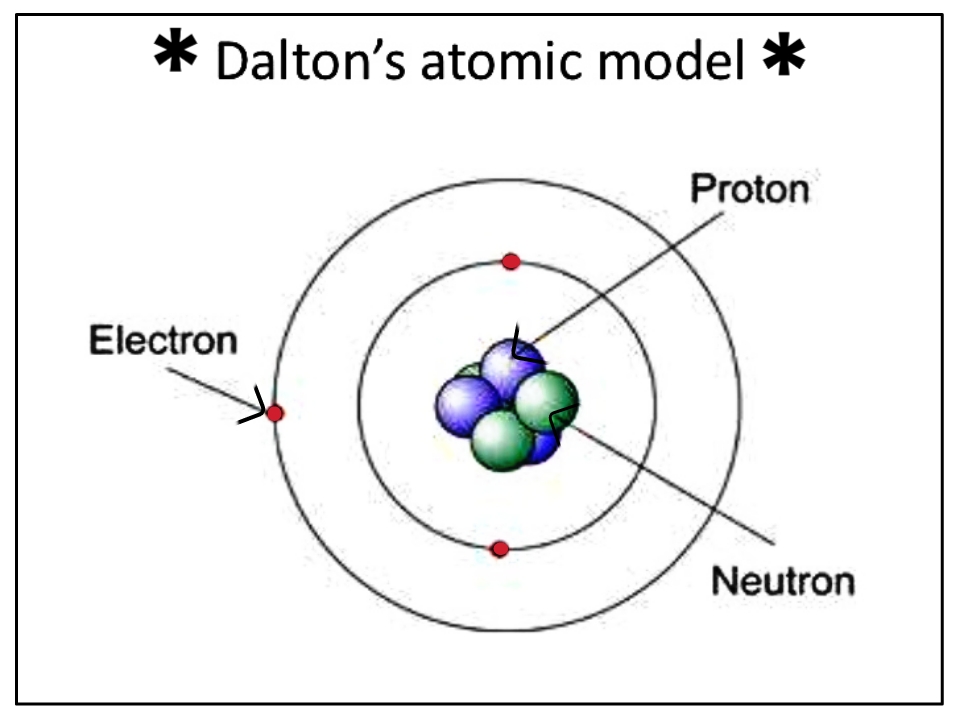
There have been many scientists that have worked on the structure of an atom, and there are several models of their findings. From way back in the early 1800s with John Dalton, through other physicists such as Ernest Rutherford, and Niels Bohr, scientists have changed the model on how an atom looks. But we simply do not know, due to not having the technology to see a single atom on it's own.
The model we currently use, and one that is most recognisable is the Bohr model. Although there have been others released since (like a parade of 3D models with the oncoming technology), we still use the Bohr model, to learn what an atom looks like.
Parts of the Atom
Nucleus: that's right, atoms have a nucleus. Like a cell in your body. But they don't house DNA or any of the organelles includes. This nuclues houses protons and neutrons. They are there to provide stability to the element the atom is made of.
Protons: protons are subatomic particles that are positively electrically charged. There is always one or more protons in the nucleus of every element. The name proton was given to it by Ernest Rutherford in 1920, and is the Greek word for first. The proton determines the atomic number, as it denotes how many protons are in the element.
Neutron: a neutron has no charge whatsoever. It remains completely neutral. It's mass is slightly larger than a proton. The sum of the amount of neutrons, called the neutron number, and atomic number (protons) together make the mass of the nucleus.
Electrons: these are parts of the atom that circle the nucleus. It is the smallest component in the atom, being 1/1386th the size of a proton. They are negatively charged, which in turn can allow for conductivity of electricity.




 Elements
Elements