Home > Sections > Elements & the Periodic Table > States of Matter
Last Updated: 14th June 2023
ARCHIVED ITEM: this page is no longer updated.
States of Matter
Keywords
Solid, liquid, gas, matter, boiling point, freezing point, atoms, ions, molecules, bonded, structure, pattern, volume.
Introduction
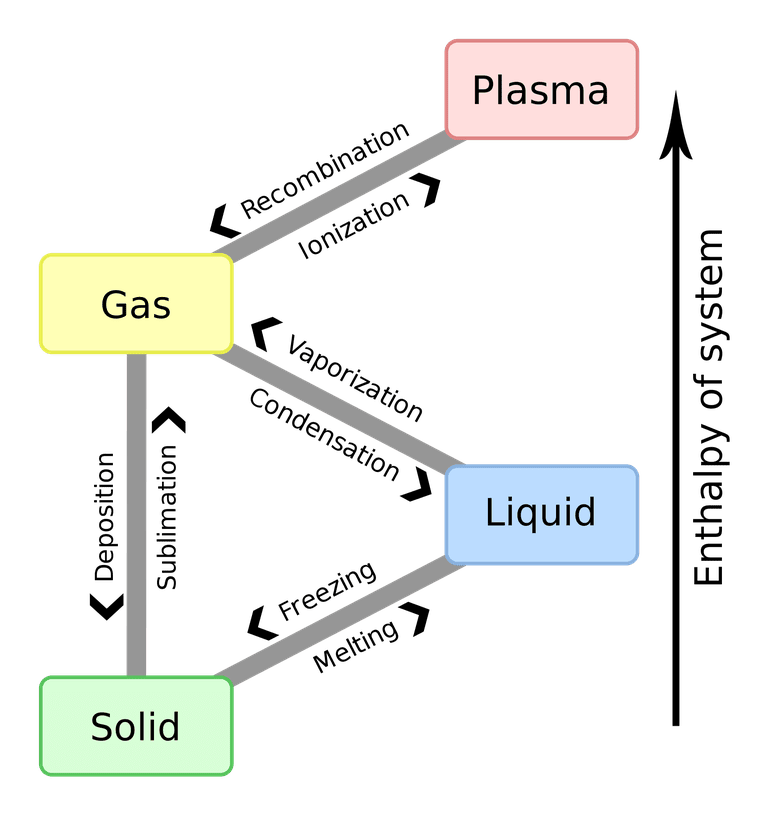
We know most elements in the world are made up of either a solid, liquid or gas. This can vary between types of elements, each having a different point where they will be solid, liquid or gas.
Solid
The main state of matter when making things, a solid is like a rock, a table, a car bonnet, and many more items. We associate a solid as being something we can hold (we can hold liquids in small quantities, but not like a solid) and that we can use to make things - I can use a hammer to put some nails in a board or two to make a shelf for example.
From a chemical makeup perspective, a solid is where particles of the element are closely bonded together, and they follow a particular shape and volume. For example, ice is formed from freezing water. It just looks like solid water, right? But look under a microscope, and it follows a pattern all along its path. This is because the pieces are fixed in one place. If you look at an element (iron, for example), it will have a similar pattern of its own, but it is solid.
Liquid
The second state of matter, liquid, is something that is in liquid form in its natural state. Water. Crude oil. It also includes man-made substances like fizzy drinks we consume, coffee, tea, petrol - a by-product of crude oil.
Again, from a chemical perspective, luiquids still maintain a fixed volume, but is so far different from a solid, as it can have a variable shape. All the molecules can move freely, which leads to spillages if not contained properly.
Gas
A gas is what is all around us. Air is a gas. But in elemental form, a single gas (oxygen for example) can have variable volume, as well as variable shape. The physical properties of a gas are neither bonded or fixed in one place. If we think of the oxygen, it can be contained in a canister and used to help people breathe it when needed in a hospital setting.
Here's a YouTube clip from Cognito to explain states of matter:
How Elements Change Matter
Let's take a couple of examples. We'll start with water. In it's natural form, it is a liquid. We drink it, and use it to clean our car, or put in a nice drink of orange squash. How do we make it solid? Well, we put it in the freezer. Water freezes and becomes a solid at 0C. If we then go the other way, we can boil it. Place ice into a kettle, and bring it up to the boil by adding heat, and at 100C water then turns to steam, also known as water vapour.
Interesting fact: ice has up to 15 different structures, depending on pressure and temperature.
When it cools, it can return to the natural state of water again. It's unique in having the same properties as either a gas, liquid or solid.
Another example is iron. In it's natural state, iron is a solid. It's heavy, and is used for construction, but we also have very small amounts in our body. To make iron a liquid, we have to heat the metal up to over 1500C.
And to make it vaporise like water does, we have to superheat it to over 2860C. This is a major contrast to what the properties are.
All elements have a boiling point, and a freezing point, where they become either a gas, or a solid.