Home > Sections > The Atmosphere > Transfer of Energy
Last Updated: 14th June 2023
ARCHIVED ITEM: this page is no longer updated.
Transfer of Energy
Keywords
Exothermic reaction, endothermic reaction, chemical, calcium oxide, snow, fire, candle, temperature, hot, cold, inward, outward.
Introduction
The transfer of energy when a chemical reaction takes place can go one of two ways - in or out. Let me explain a bit better.
Exothermic Reactions
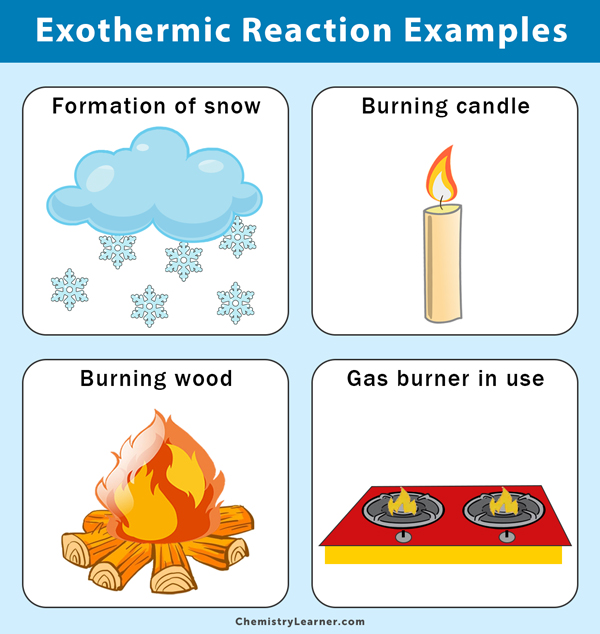
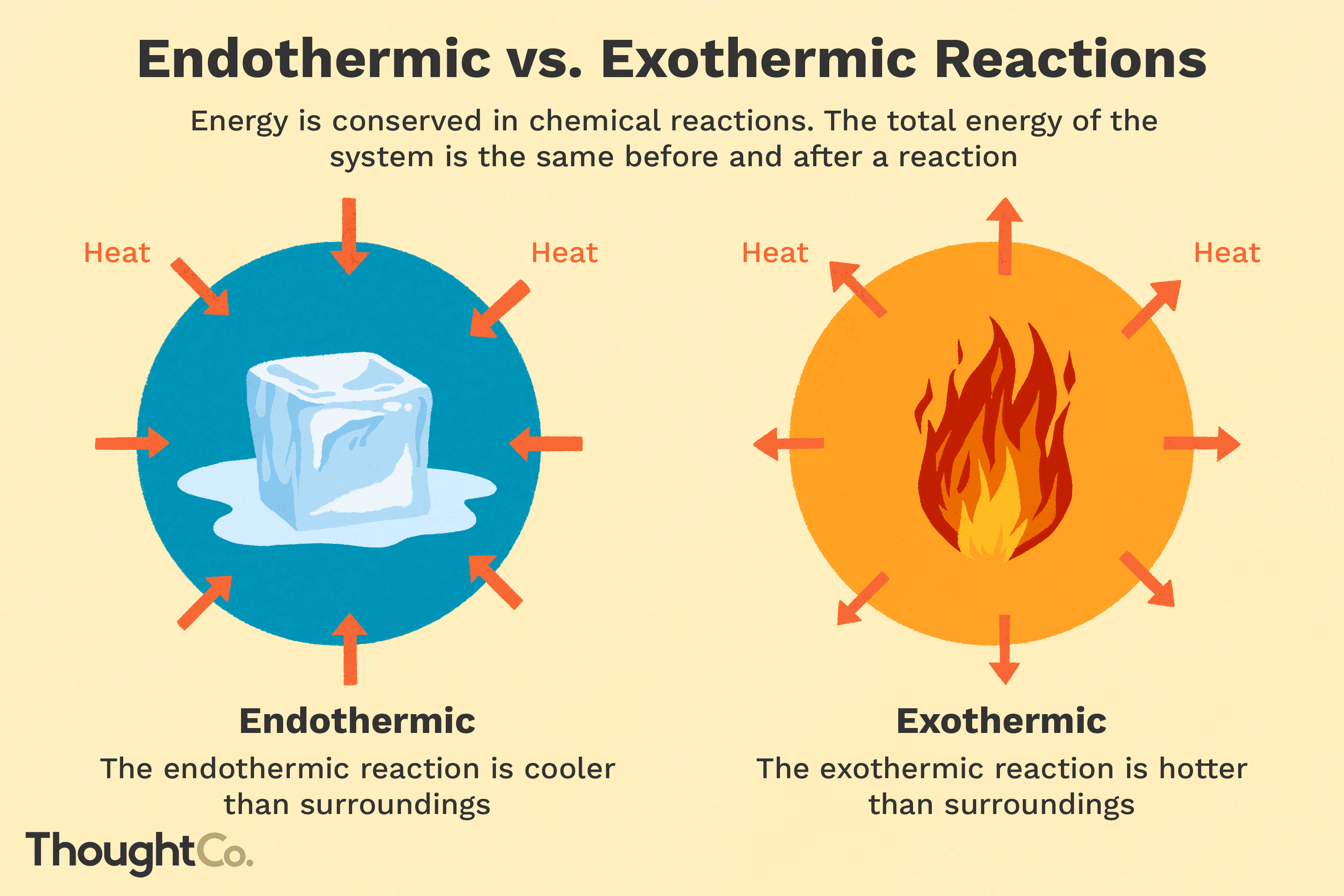
An exothermic reaction is a reaction that transfers energy to the surroundings. Outwards. This is due to an increase in thermal energy, where the item's temperature increases and it gets hotter. The majority of chemical reactions are exothermic.
Some examples of exothermic reaction include burning wood on a fire, a candle burning, the formation of snow (what? - yes, when it forms, it creates a chemical reaction that exudes heat).
Applications of Exothermic Reaction
Have you ever bought a hand warmer? You know, the ones you crack to create a chain reaction within a little pouch, and it'll keep your hands warm for hours. Yeah, that's an exothermic reaction.
There was a fad a few years ago that's not so prominent these days in the form of a can that will self-heat. You pull the ring-pull or open the can, and it causes a chain reaction that will heat the food or drink inside. It's a double layered tin can that houses the chemical within the two walls. This is usually a chemical called calcium oxide.
Interesting fact: the most easily available exothermic reaction is between detergent and water. Though it may not seem like a lot, it causes an exothermic reaction that makes heat while it washes your clothes.
Endothermic Reactions
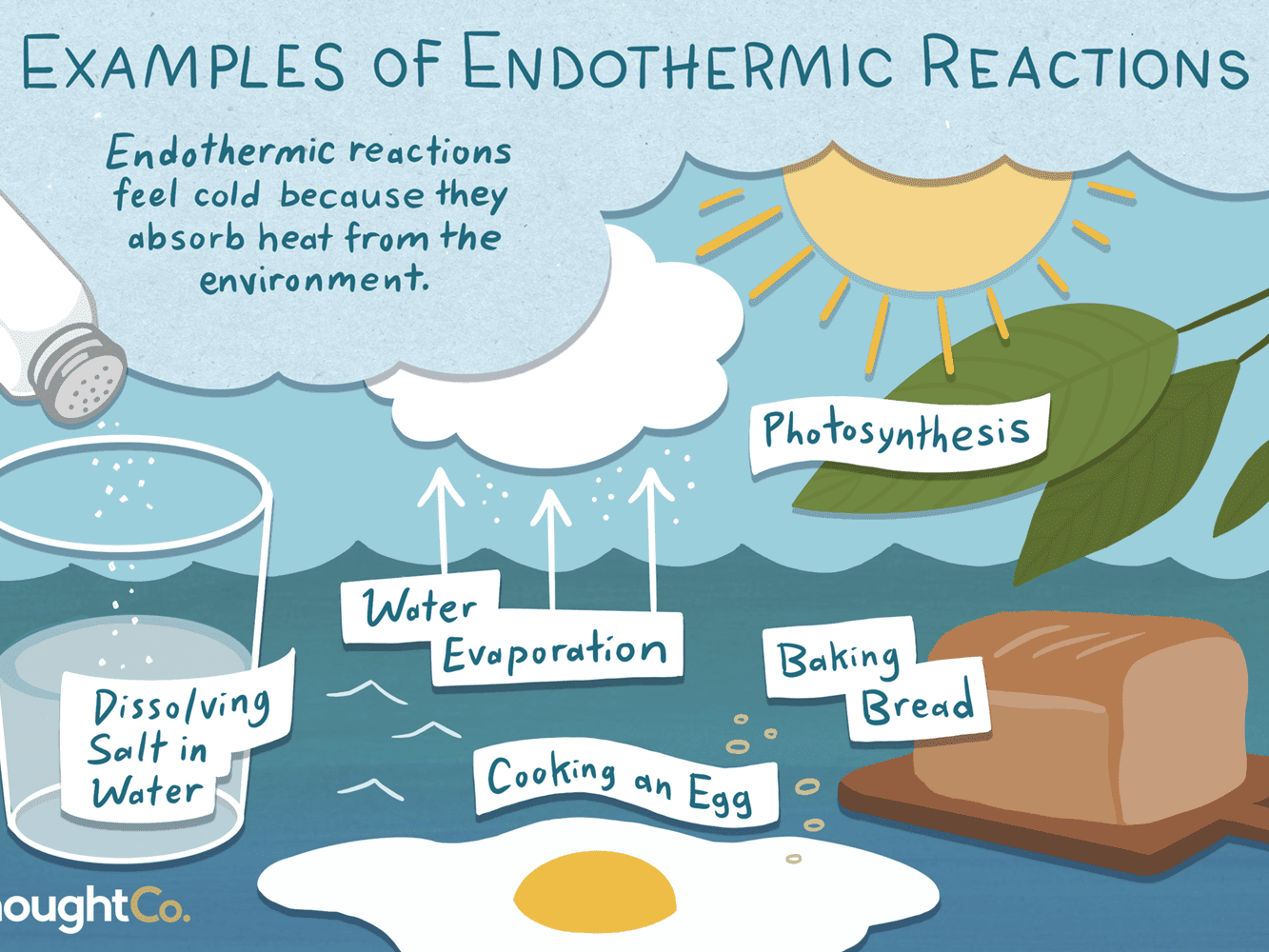
So, the opposite of an exothermic reaction is an endothermic reaction. This literally cools the area surrounding it down. With all that pulling of heat into the chemical, it makes the immediate surrounding area colder. Endothermic reactions are much less common than exothermic.
Examples of this include the reaction of acids with metal hydrocarbonates. Citric acid and sodium hydrocarbonate are in sherbert sweets (Sherbert Fountain, Dip Dab, etc) react with your mouth with an endothermic reaction.
Applications of Endothermic Reaction
One form of this is a sports injury pack that when clicked/snapped, it will cause a chain reaction within and cool. Much like the hand warmer example above, just to cool down instead.
Here is a YouTube video from Cognito on both reactions: