Home > Sections > The Atmosphere > Acid Neutralisation
Last Updated: 14th June 2023
ARCHIVED ITEM: this page is no longer updated.
Acid Neutralisation
Keywords
Acids, alkalis, bases, limewater, carbon dioxide, calcium hydroxide, foods, crystallisation, pH scale.
Introduction
You can neutralise an acid with an alkali. In similar fashion to when you place a north and a south pole together, if you have an acid solution, and you add an alkali solution to it, it brings up the pHThis means potential of Hydrogen: how concentrated the hydrogen ions are in a liquid. level from acid to more neutral.
But, how?
Testing Limewater
You can test limewater with carbon dioxide by placing some in a test tube and blowing your own air from your lungs into it using a straw. The limewater will turn cloudy. Why does it do this?
Simple. Because the carbon dioxide in your outgoing breath reacts with the limewater and turns it cloudy. The remaining chemical is called calcium hydroxide.
The equation for this reaction would be:
Limewater + carbon dioxide => calcium hydroxide
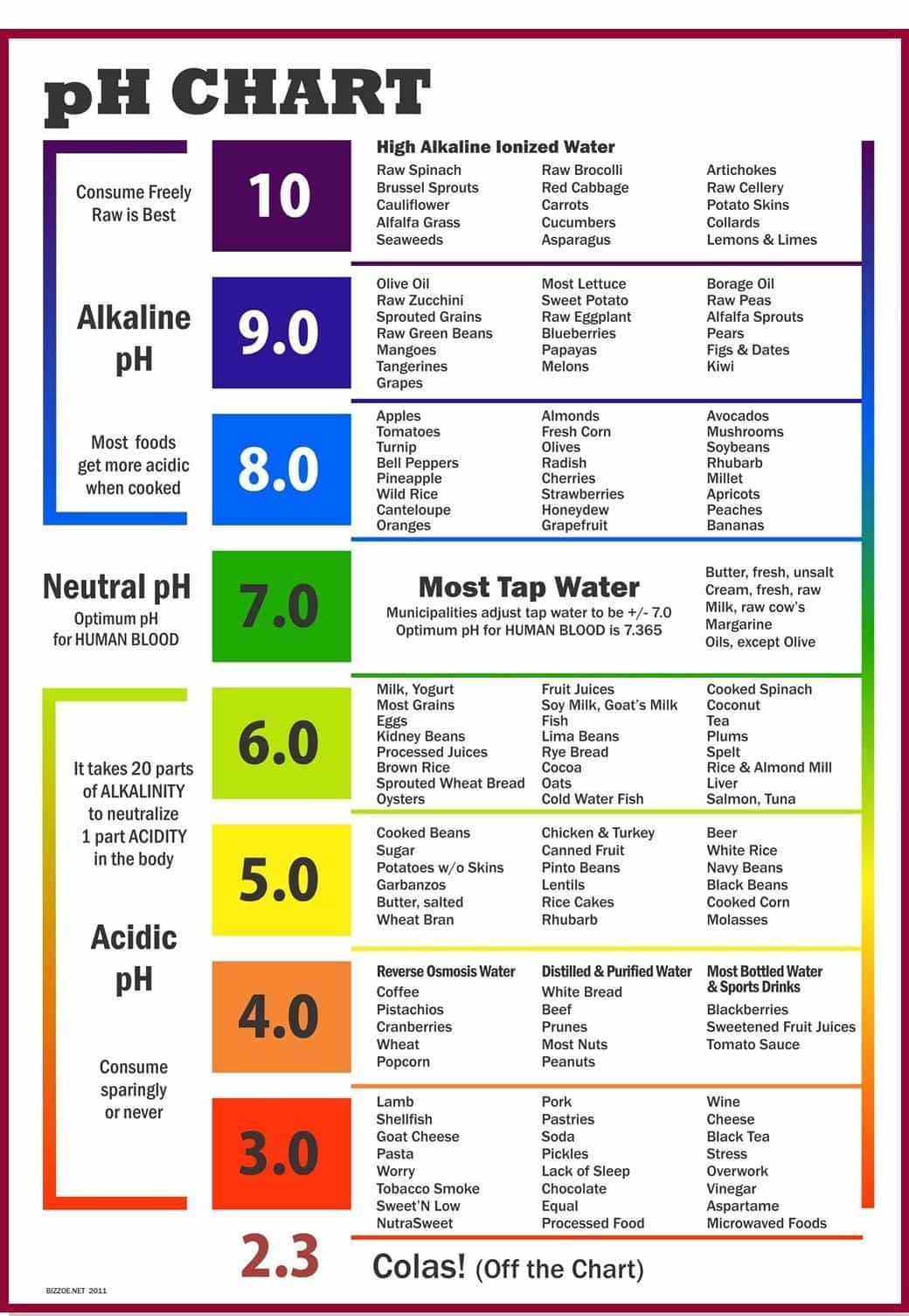
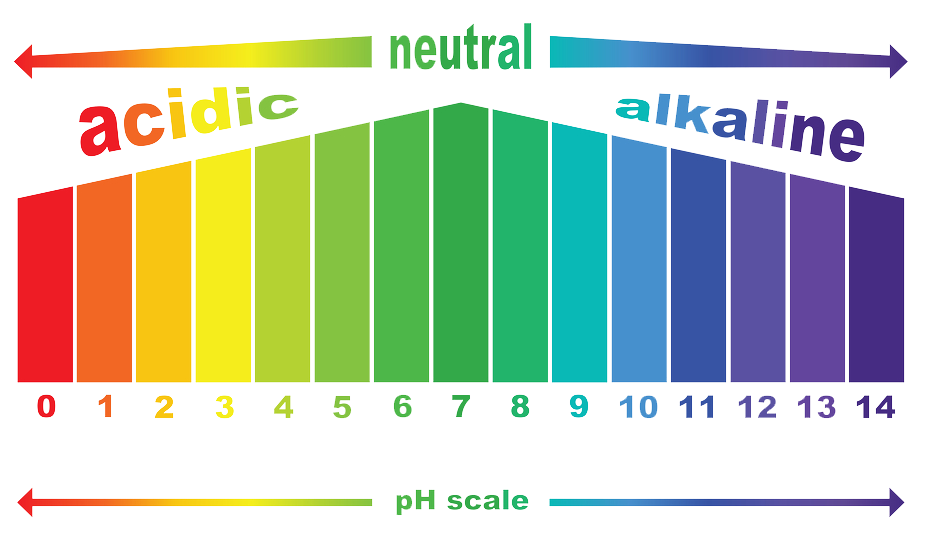
Interesting fact: you can measure the acidity or alkalinity of a liquid by using the pH scale, which runs from 0 to 14 - acid is considered being less than 7, and alkalines (bases) being more than 7.
Here is a YouTube video from Cognito on acid neutralisation:
Other Reactions
There are other areas where this type of reaction works as well. Reactions with metal oxides form a salt and water. Most metal oxides are insoluble and need to be heated to change. Adding the acid will also do this. The equation for this would be:
Metal oxide + acid => salt + water
Acids also react with metal carbonates. This reaction happens quicker than with oxides, and you can physically see them fizzing. This is because they create carbon dioxide (if you open a bottle of cola, it'll fizz - same thing to a degree!). The equation for this is:
Metal carbonate + acid => metal salt + water + carbon dioxide
One Step Further
Salt solutions can further be solidified by crystallising them. This will turn a solution into a solid. Using the technique of evaporation, adding heat to the solution will remove the solution and the salt will remain.




 The Digestive System
The Digestive System